- | 8:00 am
How human ‘organs on a chip’ can help replace animal testing
Animal testing isn’t just ethically questionable—it’s actually not that effective. Several biotech firms are developing technology that could change that.
When 4,000 beagles destined for animal testing were recently rescued from a breeding facility in Virginia, it was a reminder of the sheer scale of animals used in the pharmaceutical industry and other research: By one estimate, 192 million animals are used globally in labs each year.
Beyond the ethical challenges, the process doesn’t work very well. Of the drugs that pass animal tests, more than 90% later fail in human clinical trials. But better technology could begin to replace the use of some animals—and make drug development cheaper and more effective. And eventually, as the technology evolves, perhaps it and other alternatives could replace animal testing completely.
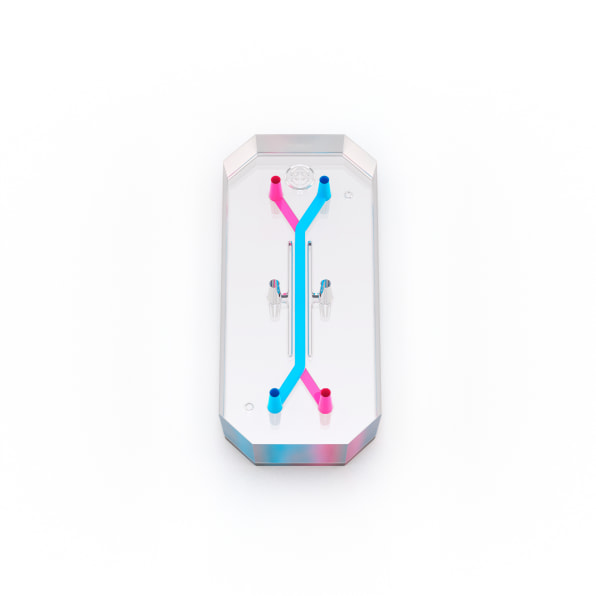
Biotech firm Emulate is one of the companies pioneering this technology. On one version of its tiny “organ on a chip,” roughly the size of a flash drive, human lung cells line two parallel channels carved into a flexible plastic. Another chip is implanted with brain cells; yet another has liver cells. The technology mimics what happens inside the body, with nutrients, air, and blood pumped through the small channels. “What we’re trying to do is recreate the simplest functional unit of every organ,” says Lorna Ewart, Emulate’s chief scientific officer.
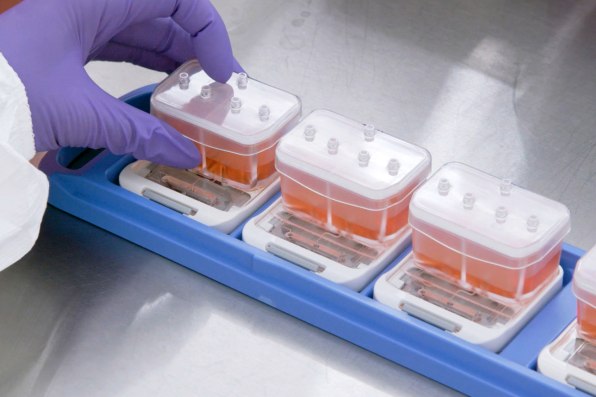
When the technology is used to test drugs, growing evidence suggests that it can work better than testing on animals. And in the case of some newer types of interventions, like gene therapy or monoclonal antibodies, animal testing currently doesn’t work at all; an organ chip could help provide critical early feedback about whether a drug is safe or effective. In its current form—and under current regulations—organ chip tech can’t fully replace animal testing. But it has the potential to significantly replace the number of animals used.
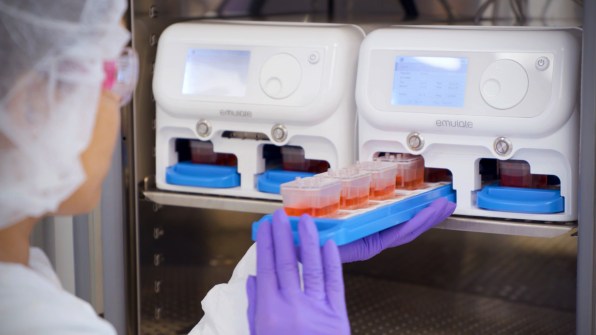
“We’ve been using these to either repurpose existing drugs that are in clinical trials or to develop new drugs using these chips, and we can do them faster and cheaper,” says cell biologist and bioengineer Donald Ingber, founding director of Harvard University’s Wyss Institute, who led a team that pioneered the first successful organ chip in 2010. In 2014, Ingber spun out Emulate, and now serves on its board.At Harvard, Ingber’s team has used the tech to identify an existing drug to treat COVID-19, which is now in clinical trials in Africa. It also developed a new treatment that shows promise in simultaneously protecting against COVID-19, the original SARS virus, multiple types of influenza, MERS, and the common cold. “We did that very quickly, and determined that it works in these human chips and in other models,” he says. “I think it has enormous potential.”
Before organ chip technology was available, drug companies had two main options for testing. “One was looking at cells in a dish, a very artificial environment,” says Ewart. “And the other, of course, is an animal model. And I think there’s lots of data that shows that, actually, those two models don’t help drug development scientists really pick the right candidate, either from a safety or efficacy perspective.”
Some drugs used to treat liver disease, for example, bind to proteins in the human liver in a way that doesn’t happen in animals; researchers didn’t see toxic effects until human trials started. Other drugs that have shown promise for treating Alzheimer’s disease in animals don’t work when they’re tested in humans. Some cancer drugs that eliminate tumors in mice don’t perform as well in humans. And the list goes on. (One Twitter account is devoted solely to pointing out hyperbolic press releases about new drugs that fail to mention that the results have only been demonstrated in mice—and therefore may be unlikely to work in humans.)
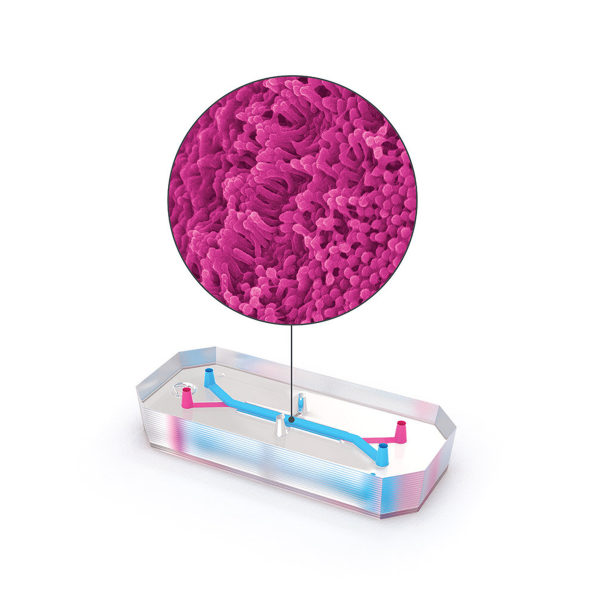
In a study currently under peer review, scientists from Emulate, along with the pharma companies Johnson & Johnson and Abbvie, found that liver chips worked much better than animals at predicting whether a particular drug would be toxic. The study looked at 27 different compounds used in drugs that made it to the market, 22 of which were later discovered to harm the liver.“Twenty-two of those drugs went through animal testing, and basically were identified as safe enough to move into clinical trials, yet later on, were either pulled from the market or required black box labeling,” says Emulate CEO Jim Corbett. (Before they were pulled, the drugs killed 208 patients and required 10 others to get liver transplants.) The liver chip tech was seven to eight times more accurate at identifying toxicity than the animal testing had been.
Organ chip technology is still at an early stage. Emulate has focused first on using the tools to test drug safety. The next step is to do more testing of efficacy, so researchers can better understand how drugs may work before clinical trials begin. The tech could be used with samples of cells from patients with rare diseases, for example, before it’s actually used in those patients directly.
To help increase use of the technology, change in regulation is one key step: A bill that recently passed the House with bipartisan support, the FDA Modernization Act, would update requirements for pharmaceutical companies that want to move a drug into clinical trials. The guidelines haven’t changed since 1938. “For the first time, this would say that you could submit alternatives to animal data,” Corbett says.
Pharma companies may also begin to use the technology more in internal drug development, since it has the potential to save some of the billions of dollars spent on drugs that don’t ultimately work. The recent study of drug toxicity on the liver calculated that if drug companies used Emulate’s liver chip alone, they could save $3 billion a year.
“Replacing animal testing for these drug companies is really hard, because you have people not wanting to take a risk and change the way they do things,” Ingber says. “But maybe the economics of it will get the attention of C-suite people.”